Nazartinib (EGF-816)
$100.00 – $700.00
Nazartinib (EGF-816) (CAS No.: 1508250-71-2), a covalent mutant-selective EGFR inhibitor, with Ki and Kinact of 31 nM and 0.222 min-1 on EGFR(L858R/790M) mutant, respectively. >98% Purity.
Synonyms: EGF-816, EGF816, EGFR inhibitor, epidermal growth factor receptor inhibitor
For Research Use Only.
- Product Details
- Description
- Additional information
- More Offers
Description
APIM050413: Nazartinib (EGF-816) is a covalent mutant-selective EGFR inhibitor, with Ki and Kinact of 31 nM and 0.222 min-1 on EGFR(L858R/790M) mutant, respectively.
CAS No.: 1508250-71-2
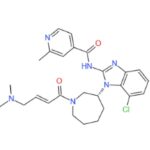
IUPAC/Chemical Name: (R,E)-N-(7-chloro-1-(1-(4-(dimethylamino)but-2-enoyl)azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide
Molecular Formula: C26H31ClN6O2
Molecular Weight: 495.02
Purity: >98% Purity
QC: Achiral and Chiral HPLCs, MS, NMR, and Quantitative Elemental Analysis Report
Solubility: Soluble in DMSO
Storage: Dry, dark and at 0 – 4 C for short term (days to weeks) or -20 C for long term (months to years).
Note: Please contact us for COA, Spectra, and SDS information.
Background Information:
The first-line treatments suggested for NSCLC patients with sensitising EGFR mutations are epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) gefitinib, erlotinib, and afatinib. Despite the impressive responses with first- and second-generation EGFR-TKIs utilised in treating patients with sensitising EGFR mutations, most patients still develop resistance. One of the expected resistance mechanisms to first- and second-generation EGFR-TKIs is the gatekeeper Thr790Met mutation found in an estimated 60% of resistant tumours. Nazartinib is a third-generation, new, permanent, oral EGFR-TKIs that selectively impedes EGFR-TKIs sensitising mutations, and Thr790Met resistance mutations are suggested as the first-line treatment of NSCLC patients with Thr790Met-positive. In some previous studies, nazartinib demonstrated clinical activity in patients suffering from Thr790Met-positive NSCLC. However, the present study will examine the clinical safety and efficacy of nazartinib in patients with EGFR-mutated NSCLC. [1]
Target: EGFR, H1975, H3255, HCC827
Ki: 31 nM
In Vitro: Nazartinib (EGF-816) has inhibitory effect on the mutant cell lines with IC50s of 4, 6, 2 nM in H1975, H3255, and HCC827, respectively, and demonstrates improved ADME and PK properties. [2] Nazartinib (EGF-816) shows potent inhibition of pEGFR levels in H3255, HCC827, and H1975 cell lines with EC50 values of 5, 1, and 3 nM, respectively. Nazartinib inhibits cell proliferation, with EC50 values of 9, 11, and 25 nM in H3255, HCC827, and H1975, respectively. Nazartinib has an OC50 (compound concentration at 50% occupancy) value of 2 and 5 nM on HCC827 and H1975, respectively. [3]
In Vivo: In H1975 mouse xenograft model, Nazartinib (EGF-816; 50 and 20 mg/kg or 25 mg/kg, p.o.) demonstrates dose-dependent efficacy with near complete tumor cells regression at the highest dose tested (50 mg/kg). [2] In H1975 mouse model, Nazartinib (EGF-816; 10 mg/kg, p.o.) induces tumor growth inhibition with a T/C (tumor/control volume) of 29%, and when doses are 30 and 100 mg/kg, tumor regressions are achieved (T/C, -61% and -80%, respectively). In the H3255 xenograft model, Nazartinib (30 mg/kg, p.o.) shows significant antitumor activity. Antiproliferative activity of Nazartinib on 89 lung cancer cell lines indicates that Nazartinib selectively inhibits cell lines containing EGFR with catalytic domain mutations. [3]
What is the solubility of Nazartinib (EGF-816) in vitro?
DMSO: > 242 mg/mL
Reference:
[1]. Jun, C. et al. “Clinical efficacy and safety of nazartinib for epidermal growth factor receptor mutated non-small cell lung cancer”, Medicine, 2021, 100, e25992
[2]. Lelais, G. et al. “Discovery of (R,E)-N-(7-Chloro-1-(1-[4-(dimethylamino)but-2-enoyl]azepan-3-yl)-1H-benzo[d]imidazol-2-yl)-2-methylisonicotinamide (EGF816), a Novel, Potent, and WT Sparing Covalent Inhibitor of Oncogenic (L858R, ex19del) and Resistant (T790M) EGFR Mutants for the Treatment of EGFR Mutant Non-Small-Cell Lung Cancers”, J. Med. Chem., 2016, 59, 6671-6689
[3]. Jia, Y. et al. “EGF816 Exerts Anticancer Effects in Non-Small Cell Lung Cancer by Irreversibly and Selectively Targeting Primary and Acquired Activating Mutations in the EGF Receptor”, Cancer Res., 2016, 76, 1591-1602
[4]. Tan, D. S.-W. et al. “Nazartinib (EGF816) in patients with treatment-naïve EGFR-mutant non-small cell lung cancer (NSCLC): Updated phase II results.”, J. Clin. Oncol. 2020, 38, DOI: 10.1200/JCO.2020.38.15_suppl.9574.
[5]. Michels, S. et al. “Abstract CT255: EATON: A phase I dose-escalation trial of nazartinib (EGF816) and trametinib in EGFR-mutant NSCLC”, Cancer Res., 2020, DOI: 10.1158/1538-7445.AM2020-CT255.
[6]. Michels, S. et al. “Abstract CT255: EATON: A phase I dose-escalation trial of nazartinib (EGF816) and trametinib in EGFR-mutant NSCLC”, Cancer Res., 2020, DOI: 10.1158/1538-7445.AM2020-CT255.
Frequent questions related to “Nazartinib (EGF-816) (CAS No. 1508250-71-2), a covalent mutant-selective EGFR inhibitor”:
Does Nazartinib bind mouse EGFR?
Who makes Nazartinib?
What is Nazartinib?
What is the mechanism of action for Nazartinib?
Popular searches related to “Nazartinib (EGF-816) (CAS No. 1508250-71-2), a covalent mutant-selective EGFR inhibitor”:
Nazartinib results
Nazartinib fda approval
Nazartinib mechanism of action
Nazartinib price
Nazartinib synthesis
Nazartinib analogue
Nazartinib structure activity study
Nazartinib in vitro
Nazartinib in vivo
Nazartinib supplier
Nazartinib clinical study
Nazartinib Cytotoxicity
Nazartinib Target
Nazartinib EGFR inhibitor
EGF-816 EGFR inhibitor
Nazartinib purchase
Nazartinib epidermal growth factor receptor inhibitor
Additional information
| Size | 10 mg, 100 mg, 5 mg, 50 mg |
|---|