ND-646
$220.00 – $1,500.00
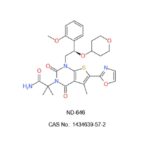
ND-646 (CAS No.: 1434639-57-2), an Orally Bioavailable and Steric Inhibitor of Acetyl-CoA Carboxylase (ACC) with IC50s of 3.5 nM and 4.1 nM for Recombinant hACC1 and hACC2, Respectively. >99% Purity.
Synonyms: Acetyl-CoA Carboxylase, ACC
For Research Use Only.
- Product Details
- Description
- Additional information
- More Offers
Description
APIM050406: ND-646 is an Orally Bioavailable and Steric Inhibitor of Acetyl-CoA Carboxylase (ACC) with IC50s of 3.5 nM and 4.1 nM for Recombinant hACC1 and hACC2, Respectively.
CAS No.: 1434639-57-2
IUPAC/Chemical Name: (R)-2-(1-(2-(2-methoxyphenyl)-2-((tetrahydro-2H-pyran-4-yl)oxy)ethyl)-5-methyl-6-(oxazol-2-yl)-2,4-dioxo-1,4-dihydrothieno[2,3-d]pyrimidin-3(2H)-yl)-2-methylpropanamide
Molecular Formula: C28H32N4O7S
Molecular Weight: 568.65
Purity: >99% Purity
QC: Achiral and Chiral HPLCs, MS, NMR, and Quantitative Elemental Analysis Report
Solubility: Soluble in DMSO
Storage: Dry, dark and at 0 – 4 C for short term (days to weeks) or -20 C for long term (months to years).
Note: Please contact us for COA, Spectra, and SDS information.
Background Information:
Cancer cells showed an extraordinary need of biological macromolecules including nucleic acids, proteins, and lipids to support continuous proliferation. Therefore, cancer cells tend to reprogram their metabolism to meet such need, resulting in alterations in glucose and glutamine metabolism. In addition, a number of cancers show an increase in de novo fatty acid synthesis (FASyn). It has been demonstrated that decrease of FASyn by genetic approaches or chemical compounds inhibited tumor growth. Acetyl-CoA carboxylase (ACC) mediates the first step of FASyn by carboxylation of acetyl-CoA to form malonyl-CoA and functions as a rate-limiting enzyme in FASyn. Two isoforms of ACC with distinct subcellular distribution and physiological roles have been identified, of which the cytosolic isoform ACC1 is predominant in control of the fatty acid synthesis, while the mitochondrial isoform ACC2 mainly regulates the fatty acid oxidation through inhibition of carnitine palmitoyltransferase I by localized malonyl-CoA production. With regard to cancer progression, current research focus is on ACC1 but not ACC2, though the both two isoforms are involved in lipid metabolism. Moreover, it was reported that ACC1 was the dominant isoform in several tested human lung cancer cell lines, while ACC2 was nearly undetectable. Upregulation of ACC1 mRNA or protein was observed in a number of cancers, including breast, liver and prostate cancers. ACC1 silencing or deletion in cancer cells led to a loss of FASyn and cell growth inhibition, which were rescued by addition of exogenous palmitate. A couple of ACC inhibitors such as 5-(tetradecyloxy)-2-furoic acid (TOFA), soraphen A and BAY ACC002 have been reported to show antitumor activity. Harriman et al. identified a series of potent and highly specific allosteric ACC dimerization inhibitors, e.g. ND-630, ND-646 and ND-654. These dimerization inhibitors bind to the key residues Arg172 (ACC1) and Arg277 (ACC2) that the AMPK-phosphorylated serine interacts with, thus mimicking the physiological inhibition of ACC dimerization and enzymatic activity by AMPK. Both ND-630 and ND-654 were shown to be liver specific. The former could reduce hepatic steatosis, improve insulin sensitivity and modulate dyslipidemia, and is currently in clinical trial phase II for treatment of nonalcoholic fat liver disease. The latter was able to suppress lipogenesis and hepatocellular carcinoma. However, ND-646, the amide derivative of ND-630, was shown to be broadly distributed, significantly inhibit fatty acid synthesis in lung tumors and strikingly suppress lung tumor growth both in vitro and in mouse models. ND-646 was well tolerated in mice. Chronic ND-646 treatment of tumor-bearing mice at the oral dose of 25 mg/kg twice daily or 50 mg/kg once daily for 31 days did not cause body weight lost, and at the higher dose of 50 mg/kg twice daily or 100 mg/kg twice daily for 6 weeks only reduced 10% body weights. [1]
ND-646 is an allosteric inhibitor of ACC, with a unique mechanism of action. It binds to the BC domain of ACC, where the AMPK phosphorylated serine of ACC interacts to prevent dimerization and activation of ACC, leading to constitutive dephosphorylation of ACC. Therefore, the phosphorylation status of ACC was used as a biomarker to evaluate how ND-646 acted. Our synthesized derivatives of ND-646 seemed to act with the same mechanism as ND-646, since compounds A2, A7 and A9 diminished the ACC phosphorylation as ND-646 did. [1]
Blockage of cancer cell FASyn by genetical or pharmacological targeting of lipogenic enzymes caused a marked decrease of lipogenesis and consequently cell growth arrest and apoptosis or autophagy depending on cancer cell types. ACC1 inhibitors ND646 (CAS No. 1434639-57-2) indeed caused A549 NSCLC growth inhibition and cell death. However, the anti-cancer mechanism of ACC1 inhibitors has not been fully elucidated. Fatty acids not only supply with building blocks for synthesis of membranes during cell division by conversion into phospholipids, but also function as signaling molecules that trigger physiological responses directly or through lipidation of proteins, e.g., WNT and Hedgehog. Therefore, ACC1 inhibition might cause comprehensive influence to cancer cells, including effects on cellular membranes and numerous signaling pathways that link to proliferation and survival. [1]
ACC1 mRNA was upregulated in NSCLC and higher ACC1 level was correlated with poor outcome in lung cancer patients, indicating ACC1 might be as a prognostic index for NSCLC patients. Moreover, we identified a series of ACC1 inhibitors with IC50 values from 2 nM to 25 nM. Some analogues of ND-646 displayed strong cancer inhibitory activity in A549 cells by impairing cell growth and inducing cell death. These compounds are worth further evaluation for NSCLC treatment. Evaluation of a new concept of combination therapy with them as well as their safety profiles will be conducted in the coming future and results will be published elsewhere. [1]
Synthetically, ND-646 and its analogues were synthesized in 15 steps from commercially available materials. [1]
Target: hACC1 and hACC2
IC50: 3.5 nM (hACC1), and 4.1 nM (hACC1)
In Vitro: ND-646 inhibits both ACC1 and ACC2 and therefore precludes the ability of ACC2 to compensate for ACC1 inhibition. ND-646 inhibits dimerization of recombinant human ACC2 BC domain (hACC2-BC) under native conditions; hACC2-BC migrates as a dimer in its absence and a monomer in its presence. In cell free systems, ND-646 inhibits enzymatic activity of recombinant human ACC1 (hACC1) with an IC50 of 3.5 nM and recombinant human ACC2 (hACC2) with an IC50 of 4.1 nM. [2]
In Vivo: To explore the impact of chronic ND-646 treatment on NSCLC tumor growth and to determine the efficacy of twice-daily dosing, athymic nude mice bearing established A549 subcutaneous tumors are treated orally with either vehicle twice daily (BID), 25 mg/kg ND-646 once daily (QD), 25 mg/kg ND-646 BID or 50 mg/kg ND-646 QD for 31 days. ND-646 at 25 mg/kg QD is ineffective at inhibiting tumor growth. However, ND-646 administered at 25 mg/kg BID or 50 mg/kg QD significantly inhibits subcutaneous A549 tumor growth. ND-646 is well tolerated throughout the treatment period, with no significant weight loss occurring after chronic ND-646 dosing, suggesting that the maximum tolerated dose (MTD) has not been reached. Mice are sacrificed at 1 hr post final dose and tissues are either prepared for immunohistochemistry (IHC) or immunoblot analysis. Tumors treated with all doses of ND-646 have lost detection of P-ACC at 1 hr, demonstrating effective tumor penetration and acute ACC inhibition by ND-646. Notably, only at the doses of ND-646 that lead to significant tumor growth inhibition (25 mg/kg BID and 50 mg/kg QD) is significant elevation of P-EIF2αS51 expression observed in tumor lysates. [2]
What is the solubility of ND-646 in vitro?
DMSO: >100 mg/mL
What is the solubility of ND-646 in vivo?
1. Solvent: DMSO, PEG300, Tween-80, and saline
Please add 10% DMSO, 40% PEG300, 5% Tween-80, and 45% saline in order; solubility ≥ 2.5mg/mL.
2. DMSO and 20% SBE-β-CD in salin
Please add 10% DMSO and 90% (20% SBE-β-CD in saline) in order; solubility 3 mg/mL.
3. DMSO and corn oil
Please add 0% DMSO and 90% corn oil in order, solubility ≥ 2.5 mg/mL.
Reference:
[1]. Li, E.-Q. et al. “Synthesis and anti-cancer activity of ND-646 and its derivatives as acetyl-CoA carboxylase 1 inhibitors”, Eur. J. Pharm. Sci., 2019, 137, 105010
[2]. Svensson, R. U. et al. “Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models”, Nat. Med. 2016, 22, 1108-1119.
[3]. Svensson, R. U. et al. “Abstract 2679: Acetyl-CoA carboxylase inhibition by ND646 reduces fatty acid synthesis and inhibits cell proliferation in human non-small cell lung cancer cells”, Cancer Res. 2014, DOI: 10.1158/1538-7445.AM2014-2679.
[4]. Harriman, G. et al. “Acetyl-CoA carboxylase inhibition by ND-630 reduces hepatic steatosis, improves insulin sensitivity, and modulates dyslipidemia in rats”, Proc. Natl. Acad. Sci. U.S.A. 2016, 3, 1520686113.
Frequent questions related to “ND-646 (CAS 1434639-57-2), an Orally Bioavailable and Steric Inhibitor of Acetyl-CoA Carboxylase (ACC)”:
Does ND-646 bind mouse ACC?
Does ND-646 bind mouse Acetyl-CoA Carboxylase?
Who makes ND-646?
What is ND-646?
What is the mechanism of action for ND-646?
Popular searches related to “ND-646 (CAS 1434639-57-2), an Orally Bioavailable and Steric Inhibitor of Acetyl-CoA Carboxylase (ACC)”:
ND-646 results
ND-646 fda approval
ND-646 mechanism of action
ND-646 price
ND-646 side effects
ND-646 ACC Inhibitor
ND-646 synthesis
ND-646 analogue
ND-646 structure activity study
ND-646 in vitro
ND-646 in vivo
ND-646 supplier
ND-646 clinical study
ND-646 ACC
ND-646 ACC inhibitor
ND-646 Acetyl-CoA Carboxylase
ND-646 Cytotoxicity
ND-646 Target
ND-646 Inhibitor
ACC
Acetyl-CoA Carboxylase
Additional information
| Size | 10 mg, 100 mg, 5 mg, 50 mg |
|---|